News
Getting Results…
-
 Read more
Read more -

UMass Chan receives $2.2 million to fund gene therapy for Cockayne syndrome
Read more -

UMass Chan researchers achieve gene therapy milestone for potential Cockayne syndrome treatment
Read more -

Top Story: UMass Chan launches Translational Institute for Molecular Therapeutics
Read more -

First gene therapy for Tay-Sachs disease successfully given to two children
Read more -

Raiden Science Foundation makes gift to UMass Chan for UBA5 gene therapy research
Read more -

UMass Chan launches Translational Institute for Molecular Therapeutics
Read more -

Sio Gene Therapies reports positive interim data for gene therapy trial
Read more -

Riaan Research Initiative funds Cockayne syndrome gene replacement therapy research at UMass Chan Medical School
Read more -

USA Today reports on first baby in gene therapy clinical trial for Sandhoff, Tay-Sachs diseases
Read more -
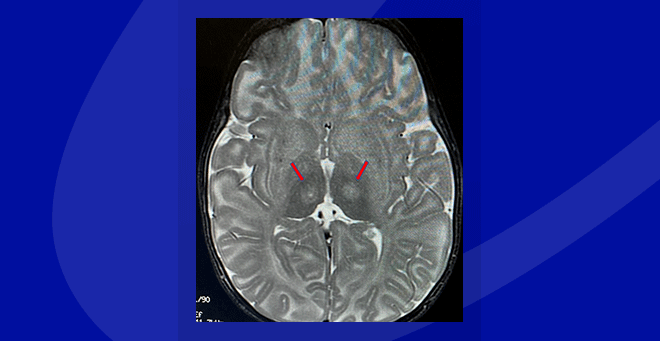
Innovative neurosurgical technique used in Tay-Sachs gene therapy clinical trial
Read more -

First patient dosed in clinical trial of gene therapy for Tay-Sachs and Sandhoff diseases
Read more -

FDA clears IND application for Tay-Sachs gene therapy
Read more