Scientists at UMass Chan Medical School have developed a technology to deliver gene therapy directly to lung tissue through intranasal administration, a development that could potentially create a new class of treatments for lung disease.
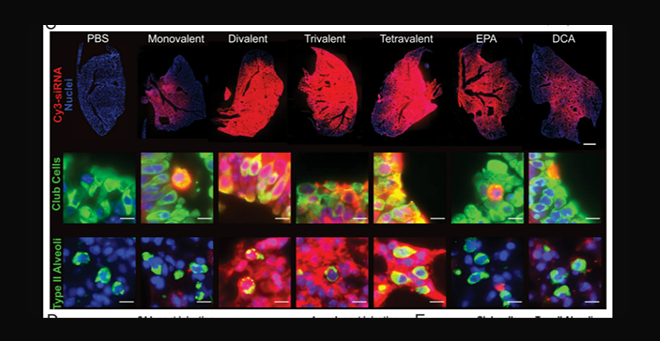
Fluoresce images of lung tissue after treatment (top, red), show local distribution of chemically modified siRNA and robust gene silencing in the lung.
Published in The Proceedings of the National Academy of Sciences, the study by a multidisciplinary team of RNA biologists, chemical biologists, immunologists and virologists describes the delivery of siRNA molecules locally to lung tissue. It is the first demonstration that multimeric siRNA molecules can be taken up into the lung after intranasal administration and achieve safe and robust genetic silencing. More importantly, the platform technology is adaptable for other pulmonary diseases such as pulmonary fibrosis and respiratory viruses.
“Achieving robust silencing at this level that is well tolerated hasn’t been achieved before,” said Vignesh N. Hariharan, PhD, co-author of the study and a postdoctoral associate in the lab of Anastasia Khvorova, PhD, the Remondi Family Chair in Biomedical Research and professor of RNA therapeutics. “We’ve shown sufficient silencing to prove anti-viral efficacy can be done with siRNA and we think this architecture is the way forward to using RNA silencing therapeutically in the lungs. This would potentially open a new class of therapeutics for treating lung diseases.”
Using the novel chemical scaffold, Dr. Hariharan and colleagues successfully delivered stabilized divalent siRNA molecules to animal models that blocked SARS-CoV-2 and prevented infection.
“The lung is a tough organ to get RNA molecules to because it’s very sensitive to potential toxins and particles that can cause immune reactions,” said Hariharan.
Small interfering RNA (siRNA) is a class of short, noncoding RNA molecules, only 20 to 24 base pairs in length, found in cells and that can be produced synthetically. They are part of the RNA interference (RNAi) system first identified by 2006 Nobel Laureate Craig Mello at UMass Chan Medical School. siRNA molecules interfere with the expression of genes by binding to messenger RNA (mRNA) after it’s been transcribed from the genome. Once bound to its target, the siRNA recruits cellular proteins that cut the mRNA, which is then degraded naturally by the cell before it can produce the corresponding protein. This prevents the cell from making proteins from that specific genetic sequence.
“If you think of the cell as being a huge block of text in a word processing program, siRNA is like a search and find function; using the right combination of letters you can find any word in the text, or in this analogy, any genetic sequence,” said Jonathan K. Watts, PhD, professor of RNA therapeutics and co-author on the study. “Using siRNA we can subvert the protein-production process by deleting specific disease-causing mRNA sequences before they are made into a protein, allowing us to treat the disease.”
In a mouse model of SARS-CoV-2, Dr. Watts and colleagues were able to deliver a siRNA that bound to and degraded viral mRNA inside the cells. The sequence targeted by researchers was found in all known variants of the SARS-Cov-2 including delta and omicron, leading the team to believe that its function was critical to the survival of the virus. Delivery of this chemically modified siRNA to animal models reduced protein translation by 60 to 80 percent, a level strong enough to prevent infection.
“Optimizing the chemical scaffold is key to the clinical application of siRNA therapeutics in lung tissue,” said Watts. “There are other delivery mechanisms, such as lipid-encased RNAs, that work well for tissues such as the liver, but this approach isn’t easy to adapt for the lung.”
By making the siRNA molecule with chemically modified nucleotides, Watts and colleagues can protect the siRNA from being degraded too quickly by the cells. This keeps the siRNA in the lung longer, and enables it to slip past the immune response.
The next step is for researchers to apply this new chemical approach to delivering siRNA to the lung for other pulmonary diseases including pulmonary fibrosis and asthma. The research team includes Dr. Khvorova, Watts, Hariharan and UMass Chan colleagues, including Jennifer Wang, MD, professor of medicine; and Katherine Fitzgerald, PhD, the Worcester Foundation for Biomedical Research Chair III, professor of medicine, associate vice provost for basic science research, vice chair of research in the Department of Medicine and director of the Program in Innate Immunity.



